
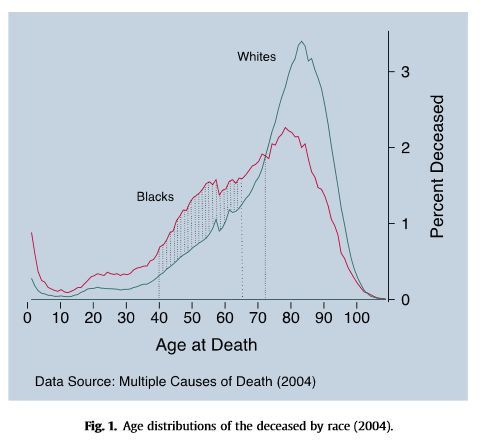
The results were dramatic: by three months after surgery, six of the seven children stopped having oculogyric crises-the distinctive eye-rolling that is a hallmark of the disease. The children ranged in age from four through nine years old at the beginning of the trial (Audrey was six at the time). The researchers injected the virus directly into each child’s brain near neurons they hoped would start making AADC and, subsequently, dopamine. Seven children participated in the trial. The gene therapy Bankiewicz and his colleagues were testing uses a harmless virus as a vector to introduce an intact version of the gene responsible for making the AADC enzyme.

Most children with the condition are unable to talk, sit up or support their own weight.Īfter years of frustration, Audrey’s parents enrolled her in a clinical trial led by Krystof Bankiewicz, a professor of neurosurgery at the University of California, San Francisco, and the Ohio State University College of Medicine. It causes severe developmental and motor disabilities, as well as sleep and mood problems. The extremely rare disorder manifests in infancy and lowers the activity of AADC, an enzyme that is critical for making the brain-signaling chemicals dopamine and serotonin. Genetic tests later diagnosed Audrey with a condition known as aromatic l-amino acid decarboxylase (AADC) deficiency, caused by mutations in a single gene.
#Scid life expectancy how to#
Finally, they confessed that they did not know how to help and sent Audrey and her parents home with a handful of pamphlets about living with a disability. Her doctors prescribed seizure medication-lots of it-which sedated her but did not stop the eye-rolling.
Despite visits to multiple specialists, no one knew what was wrong. Without warning, her body stiffened, and her eyes rolled into the corners of their sockets for hours at a time. Audrey was six months old when her parents first noticed something wasn’t right.


 0 kommentar(er)
0 kommentar(er)
